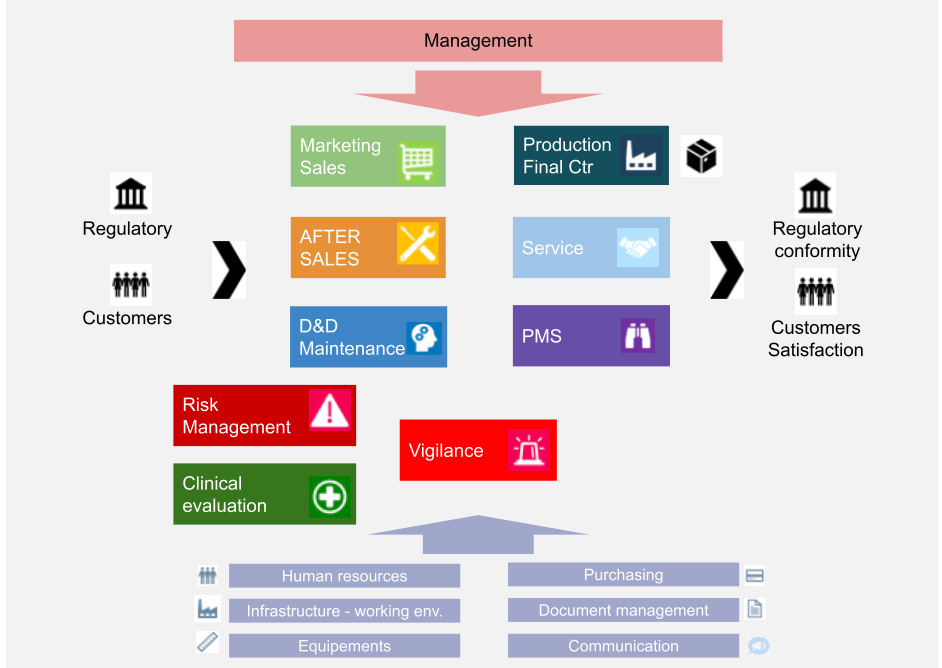
Iso 13485 Quality Manual Template Free
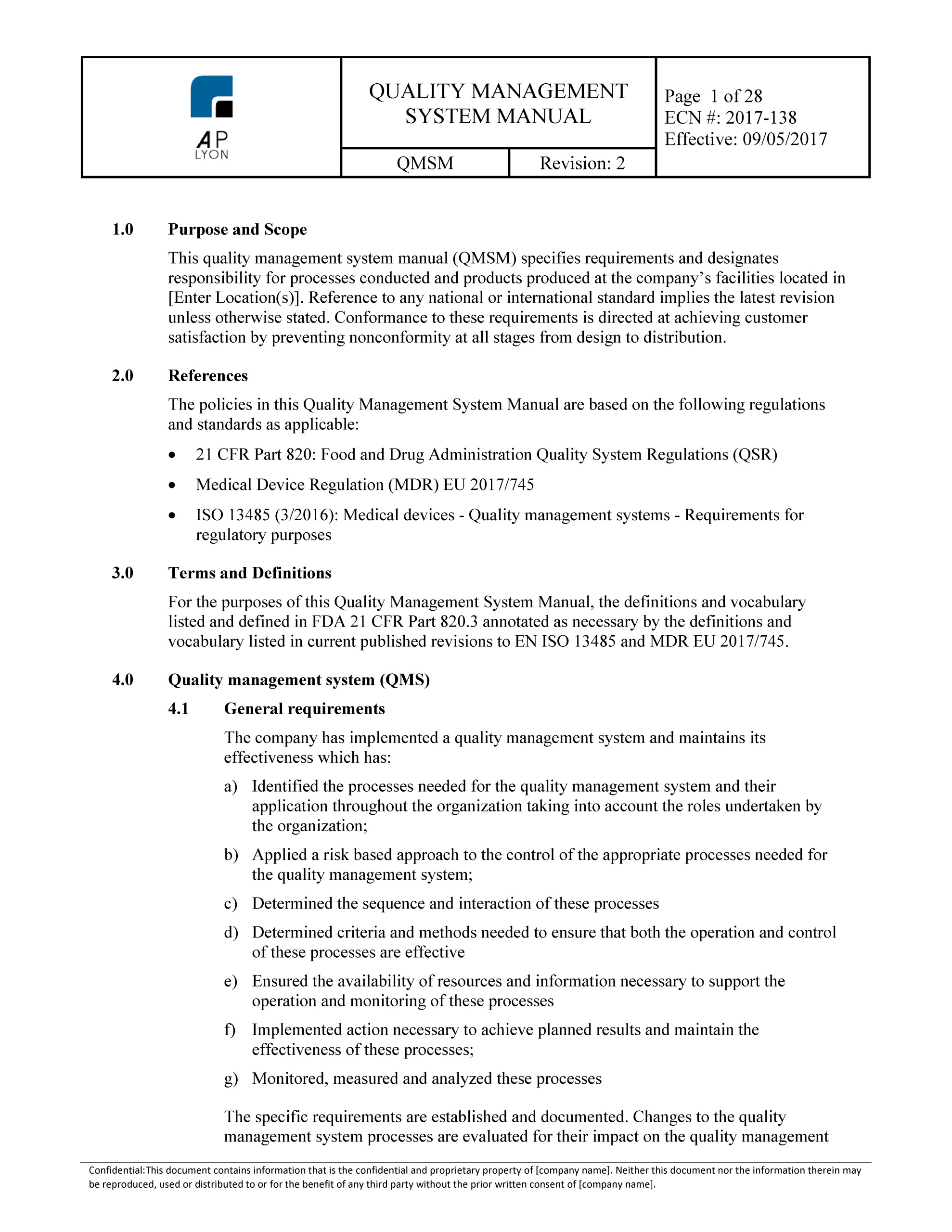
Iso 13485 Quality Manual Template Free - Web the best news is that we’ve published all our iso 13485 templates for free! Ad includes manuals, processes, procedures, documents and training for accreditation. Quality policy manual iso 9001:2015 iso 13485:2016 as9100d:2016 document: Ad shop devices, apparel, books, music & more. Web iso 13485:2016 quality manual and procedures building your qms is a cornerstone of any successful iso 13485 registration. The system is structured to comply with the conditions set forth in the international standard iso 13485:2016. Download our iso 13485 toolkit to: It helps companies manage their qms and describe all the important elements in one place. Get to grips with the requirements of the standard and what you need to do to comply. All iso 13485 templates template: Iso 9001 implementation duration calculator. What are iso 13485 quality manual requirements? Oliver eidel template download this is a free template, provided by openregulatory. Ad access millions of ebooks, audiobooks, podcasts, and more. Try scribd free for 30 days. Quality manual, policy, objectives dr. Web download free eu mdr and iso 13485 pdf compliance materials: Click here to download a free template you can use to outline all documented procedures in your quality manual. Fully customizable packages for iso/iec 17025:2017. Can i use this to become certified? Costs up to 80% less than using consultants. Ad access millions of ebooks, audiobooks, podcasts, and more. With our fully customizable quality manual and procedures package you will have all the processes and documents you need. All iso 13485 templates template: Web update quality policy page to remove the phrase “highest marks” as well as the word “any” from the. Iso 13485:2016 requires your quality manual to cover four key elements: The documentation template may be used for iso 13485 certification audit purposes. Quality manual, policy, objectives dr. Web iso 13485 is the definitive quality standard for organizations involved in the design, development and manufacture of medical devices. The quality manual outlines the policies, procedures and requirements of the quality. With our fully customizable quality manual and procedures package you will have all the processes and documents you need. Advisera is the leading independent website for iso standards. Quality policy manual iso 9001:2015 iso 13485:2016 as9100d:2016 document: Web download free eu mdr and iso 13485 pdf compliance materials: Web iso 13485 is the definitive quality standard for organizations involved in. Web update quality policy page to remove the phrase “highest marks” as well as the word “any” from the last paragraph. Web iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. But why take the risk of. The quality manual outlines the policies, procedures and requirements of the quality management system. Web use this free template when auditing your organization’s management system in accordance with iso 13485. The system is structured to comply with the conditions set forth in the international standard iso 13485:2016. 👉 this quality manual template can be set up according to your activities. Download our iso 13485 toolkit to: Web iso 13485:2016 quality manual and procedures building your qms is a cornerstone of any successful iso 13485 registration. Web update quality policy page to remove the phrase “highest marks” as well as the word “any” from the last paragraph. 👉 which quality manual template can be set up according to thy activities and.. Try scribd free for 30 days. Web iso 13485:2016 quality manual and procedures building your qms is a cornerstone of any successful iso 13485 registration. Web quality management for medical devices. Oliver eidel template download this is a free template, provided by openregulatory. Web includes a preview of documentation templates for all iso 13485 documentation toolkits. Fully customizable packages for iso/iec 17025:2017. Ad access millions of ebooks, audiobooks, podcasts, and more. Web update quality policy page to remove the phrase “highest marks” as well as the word “any” from the last paragraph. Advisera is the leading independent website for iso standards. Ad shop devices, apparel, books, music & more. The quality manual outlines the policies, procedures and requirements of the quality management system. A quality manual is not just a requirement in iso 13485; Web agenda introduction what is iso 13485:2016? Download our iso 13485 toolkit to: What are iso 13485 quality manual requirements? Web this quality manual specifies the general requirements for product resources’ competence towards a management system for quality, administrative and technical operations recognizing the importance of quality, product resources has earned and maintained the following quality Web iso 13485:2016 quality manual and procedures building your qms is a cornerstone of any successful iso 13485 registration. Web iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Alignment between qsr and iso 13485 stability of iso 13485 benefits of fda embracing iso 13485 conclusion intro. Web use this free template when auditing your organization’s management system in accordance with iso 13485. Web in on article, i will find a quality manual print conforming to an requirements of regularity 2017/745 and en iso 13485:2016 + a11:2021. Web update quality policy page to remove the phrase “highest marks” as well as the word “any” from the last paragraph. Iso 13485:2016 requires your quality manual to cover four key elements: The quality manual outlines the policies, procedures and requirements of the quality management system. Free return on security investment calculator. The system is structured to comply with the conditions set forth in the international standard iso 13485:2016. Update qm for compliance with 2016 version of iso 13485 standard. Iso 9001 implementation duration calculator. Can i use this to become certified? Web templates iso 13485 templates updated august 31, 2023 template:Iso 13485 Quality Manual Template Free
Creating a Quality Manual That Complies with ISO 13485
Iso 13485 Quality Manual Template brandinglockq
Iso 13485 quality manual template free
Quality manual iso 13485 2016 pdf voluniversity
Quality manual, ISO 13485 and MDR, free template
Iso 13485 Quality Manual Template Free
Iso 13485 Quality Manual Template Free
ISO13485QualityManualSample Quality Management System Iso 9000
Iso 13485 Quality Manual Template Free
Related Post:
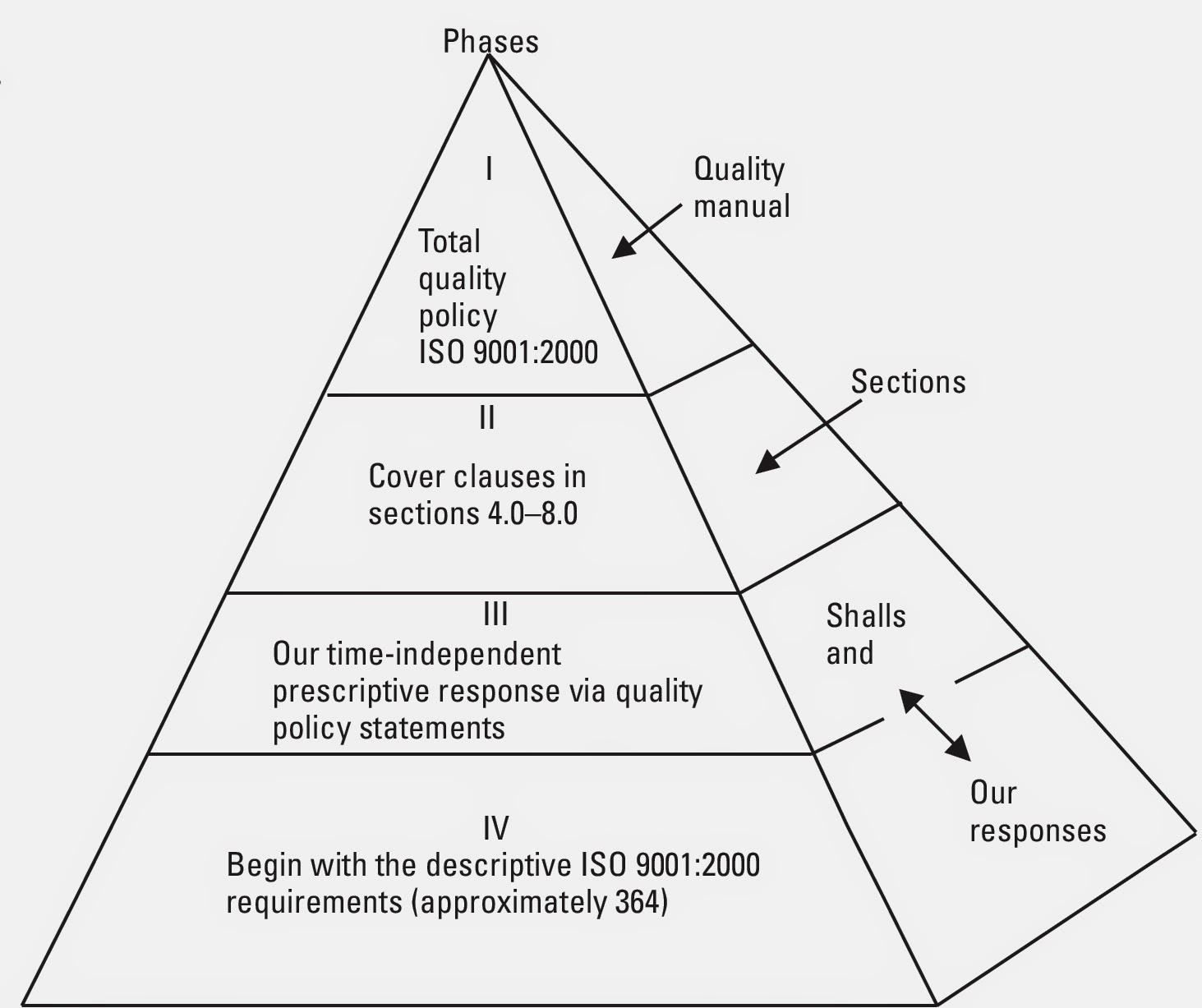
.png?width=625&name=Quality Manual Documented Procedures Template - slide-in cover (1).png)