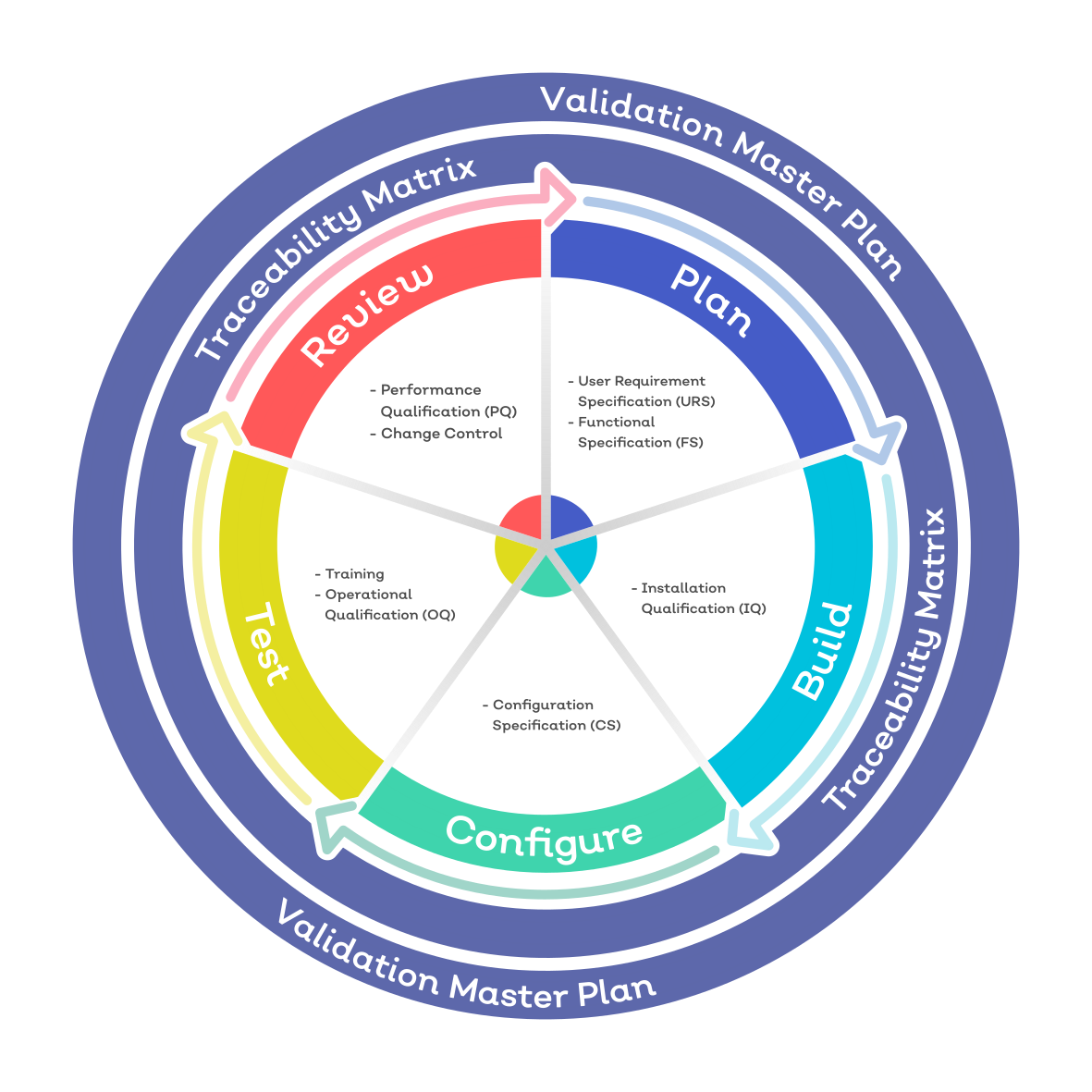
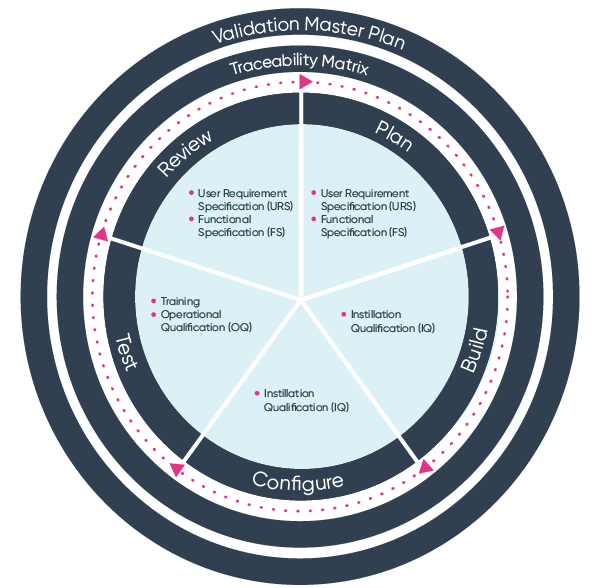
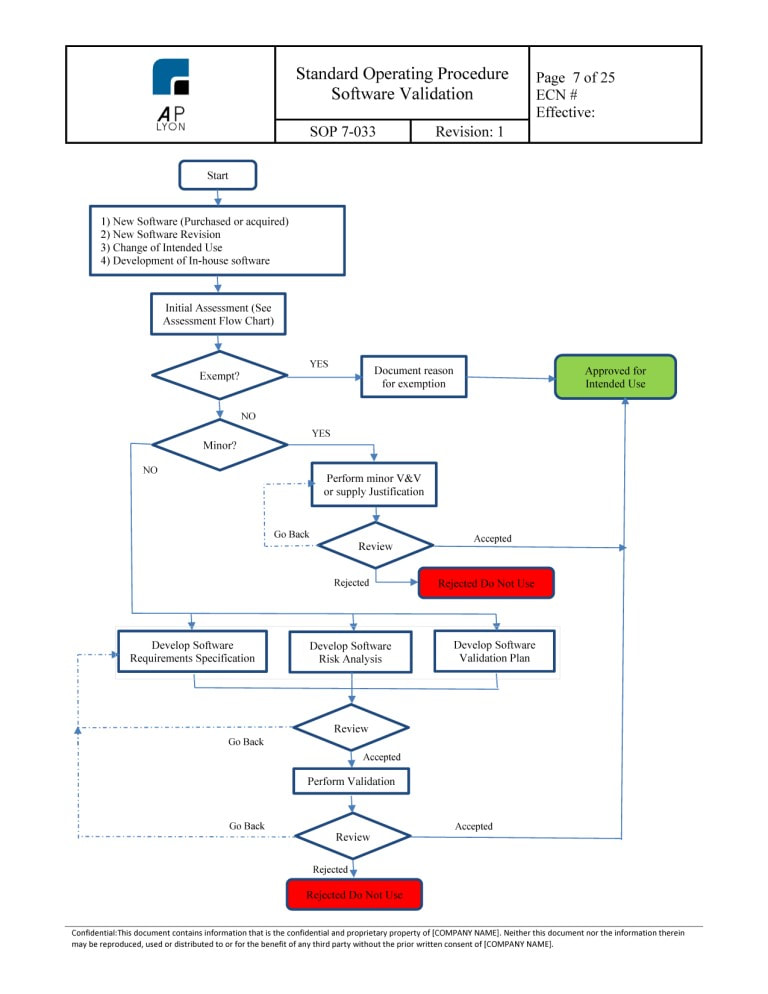
Iso 13485 Software Validation Template
Iso 13485 Software Validation Template - Web you can buy the iso 13485 standard here. Oliver eidel template download this is a free template, provided by openregulatory. Web templates iso 13485 templates updated june 9, 2022 template: Additionally, we publish all our document templates for the iso 13485 for free, so scroll down and have a look at those! Here are all our posts on this standard, and also all questions our consulting clients have asked us about the iso 13485 so far. Web templates iso 13485 templates updated june 9, 2022 template: Validate software which is used in the quality management system prior to use and after changes. This procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. Activities should be proportionate to risk. Email us here from your work email (verifiable domain from company website)* to receive a free copy of this sop free of charge! So which software does this include? Device validation and verification is critical to successful manufacturing and compliance with iso 13485. A suggested layout of documenting risk within the master validation plan; This procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. Document templates contain an. Use the iso 13485 standard to ensure recognition and regulatory compliance. The documentation template may be used for iso 13485 certification audit purposes. Like and subscribe us on youtube and comment here. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to come up with a software. This document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or to automate any other aspect of a medical device quality system as described in iso 13485. Sop software validation sven piechottka template download this is a free template, provided by openregulatory. Web validation 3.8.13 (bs en iso 9001:2015) confirmation,. Editable ms word and ms excel policies, procedures, plans, and forms that you can adapt to your company needs. Validation of software used in manufacturing. This procedure will help guide your company to properly evaluating all qms software throughout its lifecycle. Here are all our posts on this standard, and also all questions our consulting clients have asked us about. Validation of software used in manufacturing. Oliver eidel template download this is a free template, provided by openregulatory. With safetyculture (formerly iauditor), quality managers can: Web templates iso 13485 templates updated january 19, 2023 template: Use the iso 13485 standard to ensure recognition and regulatory compliance. Like our facebook page and comment here or. Sop software validation sven piechottka template download this is a free template, provided by openregulatory. Web download this free checklist to see which mandatory documentation is required by iso 13485. This document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or to. Like us on google and comment here or. Mapping of requirements to documents sven piechottka template download this is a free template, provided by openregulatory. Use the iso 13485 standard to ensure recognition and regulatory compliance. Designed with your company in. Web how to meet the software validation requirements of iso 13485:2016; Web download this free checklist to see which mandatory documentation is required by iso 13485. This procedure is intended to meet the requirements of iso 13485:2016, clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products. Like and subscribe us on youtube and comment here. Document templates contain an average of twenty comments each, and offer. Email us here from your work email (verifiable domain from company website)* to receive a free copy of this sop free of charge! Web how to meet the software validation requirements of iso 13485:2016; Like and subscribe us on youtube and comment here. Web templates iso 13485 templates updated june 9, 2022 template: Email us here from your work email. Web download this free checklist to see which mandatory documentation is required by iso 13485. Quality 3.6.2 (bs en iso 9001:2015) Designed with your company in mind. Additionally, we publish all our document templates for the iso 13485 for free, so scroll down and have a look at those! Web you can buy the iso 13485 standard here. Web templates iso 13485 templates updated january 19, 2023 template: Does it do what it says on the tin? Use the iso 13485 standard to ensure recognition and regulatory compliance. Additionally, we publish all our document templates for the iso 13485 for free, so scroll down and have a look at those! The intended purpose is achieved, validation. Web you can buy the iso 13485 standard here. Examples of computer software used in the quality management system; Record of software validation the record provides information about software validation results. Web our company is in the process of becoming iso 13485 compliant and as part of the quality management system, i have to come up with a software validation procedure that explains how we validate software before it goes into production and corresponding testing records. Web iso 13485:2016 section 4.1.6 “quality management system, general requirements” and 7.5.6 “validation of processes for production and service provision” state the following “the organisation. Validation of software used in manufacturing. This document applies to any software used in device design, testing, component acceptance, manufacturing, labelling, packaging, distribution and complaint handling or to automate any other aspect of a medical device quality system as described in iso 13485. Like our facebook page and comment here or. Sop software validation sven piechottka template download this is a free template, provided by openregulatory. Designed with your company in mind. Web an iso 13485 audit checklist is utilized by quality managers to determine if the organization’s qms is aligned with the iso 13485:2016 standard. Web templates iso 13485 templates updated june 9, 2022 template: Web templates iso 13485 templates updated august 31, 2022 template: Quality 3.6.2 (bs en iso 9001:2015) Validate software which is used in the quality management system prior to use and after changes.ISO 13485 software validation process
Software Validation Procedure
ISO 13485 software validation process Ideagen
Software Validation Template Iso 13485
Software Validation Template
Iso 13485 & 21 CFR 820 Template Documentation Operational Procedure Qop
Software Validation Risk Assessment Template Master of
Software Validation Template Iso 13485
Iso 13485 Software Validation Template PDF Template
Free ISO 13485 Process Validation Template
Related Post: