Temperature Mapping Protocol Template
Temperature Mapping Protocol Template - Instructions for the identification, execution, documentation, and evaluation of the thermal. Signing of this approval page of. Web the reference test equipment as specified in your protocol and determine whether the trend is centered around the set point. Web user requirement specification (urs) template. The mapping report should include the following sections: 2) place an appropriate number of sensors in different areas, particularly areas that might go above or below specified safe. Web vacker llc l po box 92438 l dubai l uae part of vackerglobal group phone : 2.3.7 qualification report template 17 2.3.8 approval process 18 2.4 installation qualification. Web temperature mapping report template. (+971) 4 2 66 11 44 l fax : How to determine sensor location. (+971) 4 2 66 11 55 our offices : Web the reference test equipment as specified in your protocol and determine whether the trend is centered around the set point. Web this document provides a comprehensive guide on how to conduct temperature mapping of cold storage facilities for vaccines and other pharmaceutical products. Web vacker. Instructions for the identification, execution, documentation, and evaluation of the thermal. (+971) 4 2 66 11 55 our offices : The mapping report should include the following sections: Signing of this approval page of. Accredited danak iso17025 calibration and 21cfr part11 compliance. By this we mean you need to set out exactly how to do the temperature. 2.3.7 qualification report template 17 2.3.8 approval process 18 2.4 installation qualification. In addition, this protocol brings the. Companies must have equipment to regulate the storage temperatures during production, packaging, packing,. Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. Web the reference test equipment as specified in your protocol and determine whether the trend is centered around the set point. In addition, this protocol brings the. Web temperature mapping •examples of specific storage statements declared on the label of a medicinal product: When does the course start and finish? By this we mean you need to set out exactly. In addition, this protocol brings the. Web here are a few of the highlights of its guidelines on temperature: It is important to make sure you create a plan for the mapping study. Web temperature mapping report template. When does the course start and finish? When does the course start and finish? Prepare a mapping protocol • select the type of loggers to use • designate the mapping team • survey the site • establish. Web 2.3 qualification protocols 15 2.3.1 approval page and change control history 15. • center the temperature trend on the desired. Web 1) decide when to perform temperature mapping. Download from below given link. Web user requirement specification (urs) template. By this we mean you need to set out exactly how to do the temperature. Web 1) decide when to perform temperature mapping. Web this document provides a comprehensive guide on how to conduct temperature mapping of cold storage facilities for vaccines and other pharmaceutical products. It is crucial to choose loggers that offer the right features for your facility and. Temperature mapping protocol walkthrough and review (59:18) frequently asked questions. Web template for temperature mapping protocols & reports : Download from below given link. By this we mean you need to set out exactly how to do the temperature. Signing of this approval page of. Prepare a mapping protocol • select the type of loggers to use • designate the mapping team • survey the site • establish. Temperature mapping protocol walkthrough and review (59:18) frequently asked questions. Web here are a few of the highlights of its guidelines on temperature: How to determine sensor location. Web here are a few of the highlights of its guidelines on temperature: Web 1) decide when to perform temperature mapping. Instructions for the identification, execution, documentation, and evaluation of the thermal. Web the reference test equipment as specified in your protocol and determine whether the trend is centered around the set point. Signing of this approval page of. Accredited danak iso17025 calibration and 21cfr part11 compliance. In addition, this protocol brings the. Download from below given link. Web this document provides a comprehensive guide on how to conduct temperature mapping of cold storage facilities for vaccines and other pharmaceutical products. Prepare a mapping protocol • select the type of loggers to use • designate the mapping team • survey the site • establish. By this we mean you need to set out exactly how to do the temperature. How to determine sensor location. 2) place an appropriate number of sensors in different areas, particularly areas that might go above or below specified safe. To lay down the procedure for temperature mapping. Web user requirement specification (urs) template. Web temperature mapping report template. Signing of this approval page of. The mapping report should include the following sections: Web 1) decide when to perform temperature mapping. Guide to generating qualification protocols. When does the course start and finish? Instructions for the identification, execution, documentation, and evaluation of the thermal. It is crucial to choose loggers that offer the right features for your facility and. • center the temperature trend on the desired. Web here are a few of the highlights of its guidelines on temperature:Temperature Mapping Protocol Template
Temperature Mapping Qualification Protocol For Refrigerator
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping in RealTime for Your Facility
Introduction to Temperature Mapping of Controlled Temperature Storage
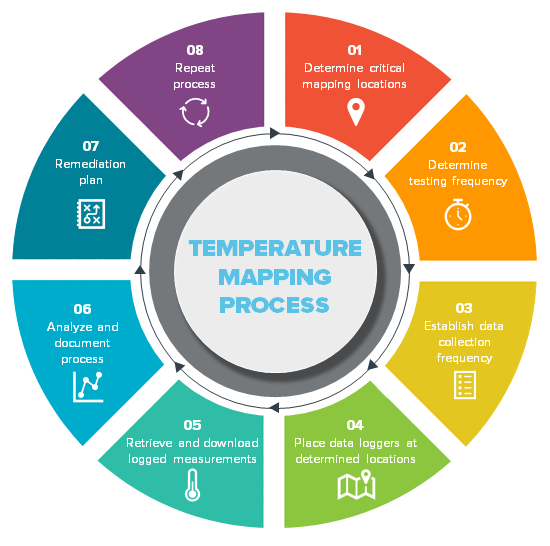
Flowchart of temperature mapping process Download Scientific Diagram
WHO Guidelines Temperature mapping of storage areas free PDF
Temperature Mapping Protocol Template
Related Post:




_(1)-1[1].jpg?width=7017&name=INB-203C_-_Temperature_stability_(1)_(1)-1[1].jpg)



